- Glucose : Production of energy through cellular respiration
- Amino Acids : Production of proteins such as enzymes ,antibodies and muscles
- Fats : Production of energy and synthesis of cellular structure
- Vitamins &
- minerals : Coenzymes which are required for cell metabolism
- Water : Reaction medium for most of the biochemical reactions
- Oxygen : Production of energy through cellular respiration
Carbon dioxide - Waste product of respiration
Oxygen - Waste product of photosynthesis
Nitrogenous wastes - From the breakdown of excessive amino acids
- E.g.urea, uric acid, ammonia
Secretions - Secreted by particular cells
- E.g. extracellular enzymes, hormones, neurotransmitter
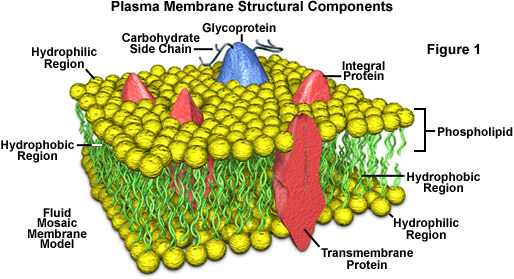
Structure of plasma membrane
1. Plasma membrane is made up of mainly phospholipid bilayer and protein molecules.2. Each phospholipid molecule consists of two parts:
a) Hydrophilic head (water loving) which made up of phosphate group.
b) Hydrophobic tail (water hating) which made up of fatty acids.
3. The fluid mosaic model proposed By Singer and Nicholson stated that the phospholipids, proteins and other components of the membrane form a dynamic and fluid structure.
4. Plasma membrane is semi-permeable because only certain substances can move across the plasma membrane.
5. The phospholipid bilayer is only permeable to
a) Small, fat soluble molecules such as fatty acids, glycerol, steroids and vitamins A,D,E,K.
b) Small uncharged molecules such as oxygen, carbon dioxide and water.
6. the proteins embedded in the phospholipid bilayer consisted of
a) Carrier protein
b) Pore protein/channel protein
7. Slightly bigger polar molecules such as glucose and amino acids cannot move across the phospholipid bilayer.These substances pas through the plasma membrane with the help of the carrier proteins.
8. Small charged mineral ions such as Na+ and K+ ions cannot move across the phospholipid bilayer. These substances pass through the plasma membrane with the help of the pore proteins.